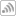Collaboration of CAS and Engineering leads to patent
Testing for heavy metal in blood, urine, soil samples, etc., is time consuming — up to weeks — and the machine is expensive — between $100,000 and $150,000. Two SIUE scientists are hoping to change this.
Edward Navarre, assistant professor of chemistry in the College of Arts and Sciences, and Brad Noble, associate professor of electrical and computer engineering in the College of Engineering, have collaborated in this project.
The project was to create a device based on a machine that is large and costly. The machine is used to determine the presence of heavy metals in all sorts of materials. Heavy metals may cause mental and physical damage to human beings. However, until now, the size and cost limits field usability of this testing.
With the Illinois budget issues, Navarre and Noble knew they had to be resourceful. Very little money went into the materials used to make their prototype.
“Many of the pieces of the device were parts that we had laying around the lab,” stated Navarre.
The patent was applied for in the names of the inventors, but SIUE is actually the owner of the device and the patent.
When the device is finalized, it will be the size of a breadbox, according to both Navarre and Noble. They stated the device will run off of a car battery and will yield results within minutes. Training for the device would take place in a matter of hours.
Navarre explains the technique:
“The lamp on the far left produces a spectrum of light [seen as red in above picture] which is specific to one element, and that is the element we are going to analyze. So that light centers the lens to focus it on this filament [seen as bright light in center]. The sample is put on the filament, evaporated and then vaporized and atomized We make a little cloud of those atoms — the atoms in gas phase. The light coming in — since these are all the same types of atoms — they can absorb some of that light. Then it refocuses the beam of light on the other side of the filament and detects it. The decrease in light is proportional to the concentration of atoms in the cloud. So we have a direct proportion between the amount of light absorbed and the concentration of that element that we put on the element.”
Below are some questions posed to the inventors with their responses. (ECN – Navarre and BN – Noble)
Questions: What prompted you to devise your invention? Were there discussions between the colleges?
ECN: For me, the device comes from two sources. First, the overall theme of my research is to make atomic spectroscopy instruments smaller, more portable, and less expensive. Second, after doing restoration work on a house last year, I needed to be tested for accidental lead poisoning. The results took more than 3 days to arrive. The application of my research to lead in blood was a natural response to that experience. The collaboration grew out of knowing each other via a colleague (Dr. Michael Shaw). Brad introduced me to microcontrollers and it seemed to be a perfect solution to what I was trying to do. My research had a “nail” and Brad showed up with a very elegant “hammer”.
BN: I have collaborated with Dr. Mike Shaw in Chemistry for several years, specifically in enhancing the instrumentation methods and techniques he uses in his research. I met Ed through Mike and much of Ed’s research involves developing new instrumentation methods for Chemistry so extending my collaboration with him was only natural. It has been a pleasure to work with both of them and I find the work extremely interesting and challenging. Where possible, we try to get our students involved in our work. Chris Edmiston, one of my electrical engineering students, worked on developing the power supply controller that is in the atomic spectrometer we’re using in the blood analyzer as a URCA project.
Questions: How will this be applied in the field? What are the practical applications?
ECN: One application focus is clinical analysis of blood and urine for metals, be they toxic or nutritive. For clinical use, we need to be able to encapsulate sufficient spectrometric knowledge in the device that the user needs little training and almost no detailed understanding of atomic spectrometry. One goal is to have nurses operate the instrument after one hour of training. The advantage of having an inexpensive instrument widely diffused in hospital and clinical settings is that results are available almost immediately.
Some applications of a portable atomic spectrometer are apparent in field analysis. The instrument is particularly appropriate for water analysis. An instrument that can be brought to the sample can reduce the challenges associated with sampling and preserving water samples from distant locations.
Finally, an atomic spectrometer that is inexpensive can have an important impact on education. Several times I have had foreign students comment to me that in their home country analytical instruments are scarce. The same can be said for many small 4-year colleges in the U.S. Hopefully this project can overcome some of the economic barriers to teaching experimental atomic spectrometry.
Question: How soon are you hoping to have the final, marketable version?
ECN: Given abundant funding and full-time work on the project, we have estimated that it would require 2 years to bring this idea to a field-ready device. In the absence of those two factors, several years of work and a lot of student research training will be invested in the device both from Chemistry and Electrical Engineering.
Question: Is this something that you will be able to use for class or field research at SIUE?
ECN: Speaking only for the Chemistry department, it can be put to use in a couple of Chemistry laboratory courses. I can envision uses in the Environmental Sciences program, as well. I hope to be able to involve the SIUE School of Nursing in the implementation of clinical testing.
BN: In Electrical and Computer Engineering, it is unlikely we will have any class or field applications for the device, however the technologies the device uses are certainly appropriate to teach and discuss in some of our classes. It is possible our Civil Engineering department might be able to use it in their environmental engineering classes but I have not consulted with them about this yet.
Question: Do you plan on using the process as training tools for your students?
ECN: Not only is this a training tool, the development of the device and the measurement methods for it will be student projects. We have already had the participation of one Electrical Engineering student, Chris Edmiston, who designed the microcontroller feedback and machine-human interface. Currently, one of my students, Sean Hudson, is finishing his masters thesis on developing methods for analyzing blood samples using the device. Leonard Bright, who completed his MS degree in September, developed a paint sampling method which is specifically adapted to the device.
Filed Under: Chemistry